Recombinant DNA
Recombinant DNA (rDNA) is a form of artificial DNA that is created by combining two or more sequences that would not normally occur together.[1] In terms of genetic modification, it is created through the introduction of relevant DNA into an existing organismal DNA, such as the plasmids of bacteria, to code for or alter different traits for a specific purpose, such as antibiotic resistance.[1] It differs from genetic recombination in that it does not occur through natural processes within the cell, but is engineered.[1] A recombinant protein is a protein that is derived from recombinant DNA.[2]
The recombinant DNA technique was first proposed by Peter Lobban, a graduate student, with A. Dale Kaiser at the Stanford University Department of Biochemistry. The technique was then realized by Lobban and Kaiser; Jackson, Symons and Berg; and Stanley Norman Cohen, Chang, Herbert Boyer and Helling, in 1972–74. They published their findings in papers including the 1972 paper "Biochemical Method for Inserting New Genetic Information into DNA of Simian Virus 40: Circular SV40 DNA Molecules Containing Lambda Phage Genes and the Galactose Operon of Escherichia coli", the 1973 paper "Enzymatic end-to-end joining of DNA molecules" and the 1973 paper "Construction of Biologically Functional Bacterial Plasmids in vitro",[3] all of which described techniques to isolate and amplify genes or DNA segments and insert them into another cell with precision, creating a transgenic bacterium.
Recombinant DNA technology was made possible by the discovery, isolation and application of restriction endonucleases by Werner Arber, Daniel Nathans, and Hamilton Smith, for which they received the 1978 Nobel Prize in Medicine. Cohen and Boyer applied for a patent on the Process for producing biologically functional molecular chimeras which could not exist in nature in 1974. The patent was granted in 1980.
Contents |
Applications and methods
Cloning and relation to plasmids
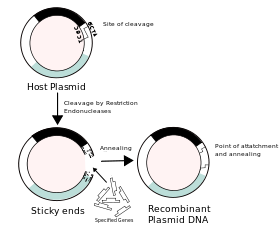
The use of cloning is interrelated with recombinant DNA in classical biology, as the term "clone" refers to a cell or organism derived from a parental organism,[1] with modern biology referring to the term as a collection of cells derived from the same cell that remain identical.[1] In the classical instance, the use of recombinant DNA provides the initial cell from which the host organism is then expected to recapitulate when it undergoes further cell division, with bacteria remaining a prime example due to the use of viral vectors in medicine that contain recombinant DNA inserted into a structure known as a plasmid.[1]
Plasmids are extrachromosomal self-replicating circular forms of DNA present in most bacteria, such as Escherichia coli (E. Coli), containing genes related to catabolism and metabolic activity,[1] and allowing the carrier bacterium to survive and reproduce in conditions present within other species and environments. These genes represent characteristics of resistance to bacteriophages and antibiotics[1] and some heavy metals, but can also be fairly easily removed or separated from the plasmid by restriction endonucleases,[1], which regularly produce "sticky ends" and allow the attachment of a selected segment of DNA, which codes for more "reparative" substances, such as peptide hormone medications including insulin, growth hormone, and oxytocin. In the introduction of useful genes into the plasmid, the bacteria are then used as a viral vector, which are encouraged to reproduce so as to recapitulate the altered DNA within other cells it infects, and increase the amount of cells with the recombinant DNA present within them.
The use of plasmids is also key within gene therapy, where their related viruses are used as cloning vectors or carriers, which are means of transporting and passing on genes in recombinant DNA through viral reproduction throughout an organism.[1] Plasmids contain three common features—a replicator, selectable marker and a cloning site.[1] The replicator or "ori"[1] refers to the origin of replication with regard to location and bacteria where replication begins. The marker refers to a particular gene that usually contains resistance to an antibiotic, but may also refer to a gene that is attached alongside the desired one, such as that which confers luminescence to allow identification of successfully recombined DNA.[1] The cloning site is a sequence of nucleotides representing one or more positions where cleavage by restriction endonucleases occurs.[1] Most eukaryotes do not maintain canonical plasmids; yeast is a notable exception.[4] In addition, the Ti plasmid of the bacterium Agrobacterium tumefaciens can be used to integrate foreign DNA into the genomes of many plants. Other methods of introducing or creating recombinant DNA in eukaryotes include homologous recombination and transfection with modified viruses.
Chimeric plasmids

When recombinant DNA is then further altered or changed to host additional strands of DNA, the molecule formed is referred to as "chimeric" DNA molecule,[1] with reference to the mythological chimera, which consisted as a composite of several animals.[1] The presence of chimeric plasmid molecules is somewhat regular in occurrence, as, throughout the lifetime of an organism[1], the propagation by vectors ensures the presence of hundreds of thousands of organismal and bacterial cells that all contain copies of the original chimeric DNA.[1]
In the production of chimeric(from chimera) plasmids, the processes involved can be somewhat uncertain[1], as the intended outcome of the addition of foreign DNA may not always be achieved and may result in the formation of unusable plasmids. Initially, the plasmid structure is linearised[1] to allow the addition by bonding of complementary foreign DNA strands to single-stranded "overhangs"[1] or "sticky ends" present at the ends of the DNA molecule from staggered, or "S-shaped" cleavages produced by restriction endonucleases.[1]
A common vector used for the donation of plasmids originally was the bacterium Escherichia coli and, later, the EcoRI derivative[5], which was used for its versatility[5] with addition of new DNA by "relaxed" replication when inhibited by chloramphenicol and spectinomycin, later being replaced by the pBR322 plasmid.[5]In the case of EcoRI, the plasmid can anneal with the presence of foreign DNA via the route of sticky-end ligation, or with "blunt ends" via blunt-end ligation, in the presence of the phage T4 ligase [5], which forms covalent links between 3-carbon OH and 5-carbon PO4 groups present on blunt ends.[5] Both sticky-end, or overhang ligation and blunt-end ligation can occur between foreign DNA segments, and cleaved ends of the original plasmid depending upon the restriction endonuclease used for cleavage.[5]
Synthetic insulin production using recombinant DNA
One breakthrough in recombinant DNA technology was the manufacture of biosynthetic "human" insulin, which was the first medicine made via recombinant DNA technology ever to be approved by the FDA. Insulin was the ideal candidate because it is a relatively simple protein and was therefore relatively easy to copy, as well as being extensively used to the extent that if researchers could prove that biosynthetic "human" insulin was safe and effective, the technology would be accepted as such, and would open opportunities for other products to be made in this fashion.
The specific gene sequence, or oligonucleotide, that codes for insulin production in humans was introduced to a sample colony of E. coli (the bacteria found in the human intestine). Only about 1 out of 106 bacteria picks up the sequence. However, because the lifecycle is only about 30 minutes for E. coli, this limitation is not problematic, and in a 24-hour period, there may be billions of E. coli that are coded with the DNA sequences needed to induce insulin production.[6]
However, a sampling of initial reaction showed that Humulin was greeted more as a technological rather than a medical breakthrough, and that this sentiment was building even before the drug reached pharmacies.
The Economist concluded: "The first bug-built drug for human use may turn out to be a commercial flop. But the way has now been cleared-and remarkably quickly, too—for biotechnologists with interesting new products to clear the regulatory hurdles and run away with the prizes."[7]
Ultimately, widespread consumer adoption of biosynthetic "human" insulin did not occur until the manufacturers removed highly-purified animal insulin from the market, thereby leaving consumers with no other alternative to synthetic varieties.
See also
- Asilomar conference on recombinant DNA
- Ice-minus bacteria
- List of recombinant proteins
- Recombinant virus
- Transgenic bacteria
- Vector DNA
Notes
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 Jeremy M. Berg; John L. Tymoczko; Lubert Stryer (2007). Biochemistry. San Francisco: W. H. Freeman. ISBN 0-7167-8724-5.
- ↑ The Recombinant Protein Handbook
- ↑ Cohen SN, Chang AC, Boyer HW, Helling RB (1973). "Construction of biologically functional bacterial plasmids in vitro". PNAS 70 (11): 3240–3244. doi:10.1073/pnas.70.11.3240. PMID 4594039.
- ↑ "Plasmids in eukaryotic microbes: an example". http://www.sci.sdsu.edu/~smaloy/MicrobialGenetics/topics/plasmids/yeast-plasmid.html. Retrieved 2007-06-05.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Nathan P. Kaplan, Nathan P. Colowick, Ray Wu (1980). Recombinant DNA, Volume 68: Volume 68: Recombinant Dna Part F (Methods in Enzymology). Academic Press. ISBN 0-1218-1968-X.
- ↑ Human insulin from recombinant DNA technology - Johnson 219 (4585): 632 - Science
- ↑ Invisible Frontiers: The Race to Synthesize a Human Gene"(1987, Tempus Books of Microsoft Press)
References
- Garret, R. H.; Grisham, C. M. (2000). Biochemistry. Saunders College Publishers. ISBN 0030758173.
- Colowick, S. P.; Kapian, O. N. (1980). Methods in Enzymology - Volume 68; Recombinant DNA. Academic Press. ISBN 012181968X.
- Inoue, Noboru; Takeuchi, Hideya; Ohashi, Makoto; Suzuki, Takamoto; Makoto Takeuchi (1995). "The production of recombinant human erythropoietin". Biotechnology Annual Review (Elsevier Science B.V.) 1: 297–300. ISBN 9780444818904.
External links
- Fact Sheet Describing Recombinant DNA and Elements Utilizing Recombinant DNA Such as Plasmids and Viral Vectors, and the Application of Recombinant DNA Techniques in Molecular Biology
- Plasmids in Yeasts
- A 3D animation illustrating the process by which a protein is mass-produced using spliced DNA and bacterial replication
- Recombinant DNA research at UCSF and commercial application at Genentech Herbert W. Boyer, Living history project. Oral history.
|
||||||||||||||||||||||||||||||||||||||||||||